Krypton-85
Krypton-85 is a radioisotope of krypton, simply meaning it is a radioactive isotope. This specific isotope will decay into stable rubidium-85. It has 36 protons, 36 electrons, and 48 neutrons, with a mass number of 85. It is found in the atmosphere as a gas or can be a by-product in nuclear explosions, volcanoes, earthquakes, and more. Properties are: tasteless, odorless, colorless, and produces some beta and gamma rays. It belongs to the group of noble gases and has a lot of uses.
Krypton-85 is used for leak detection, plasma displays, light bulbs, spark gaps and more! The leak detection method is used on a lot of things, varying to cars, to silicon MEMs (Micro-Electro-Mechanical Systems). Whatever they are testing is placed into a pressurized room with a air mixture containing krypton-85. If krypton is able to make its way into the object, it has a leak in it. This method was developed in the 1950s.
Video on krypton-85: http://www.youtube.com/watch?v=Y2CAmZ_Uk4w
Work sited
"Chemistry Explained." Krypton, Chemical Element. N.p., n.d. Web. 08 Dec. 2013.
"Krypton-85 Radioisotope Leak Testing." Krypton-85 Leak Testing Method. N.p., n.d. Web. 10 Dec. 2013.
"Krypton-85." Lindepremiumproducts. N.p., n.d. Web. 10 Dec. 2003.
"College of Engineering." Penn State Department of Architectural Engineering. N.p., n.d. Web. 10 Dec. 2013.
 |
| Diagram of a pressurized room |
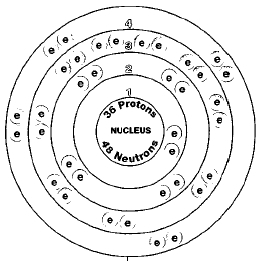 |
| Bohr model of krypton-85 |
